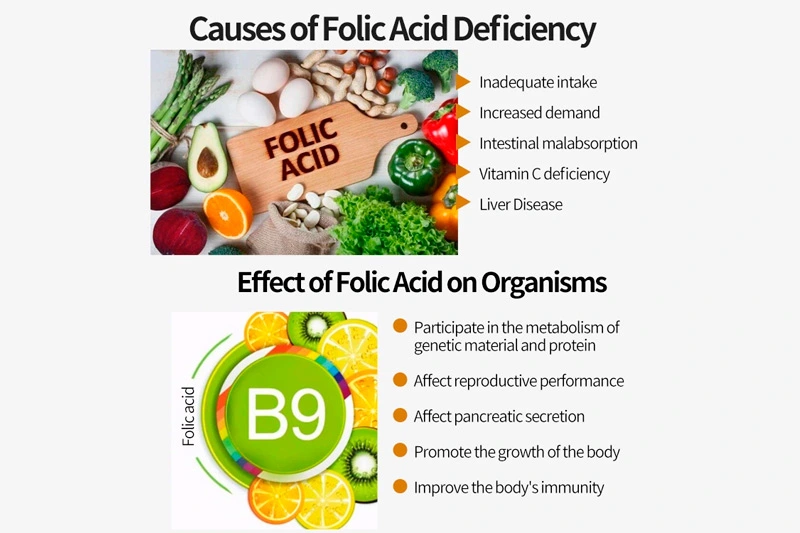
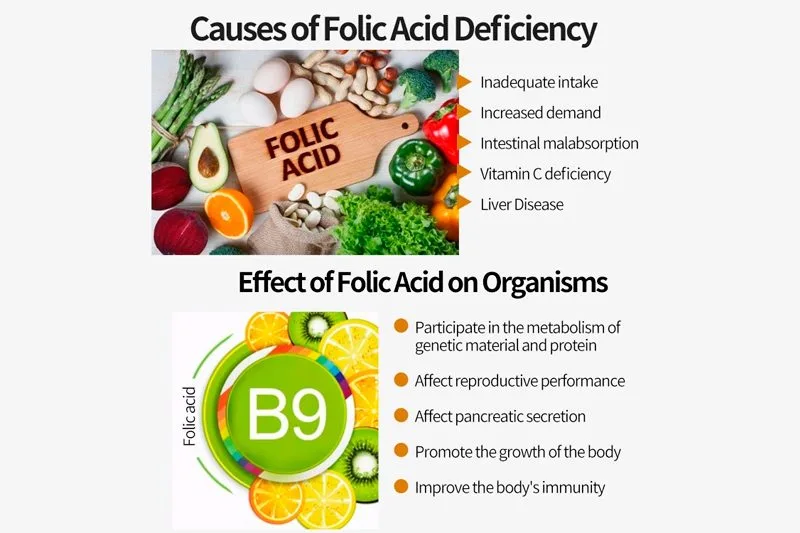
In the intricate world of pharmaceuticals, the production and utilization of active pharmaceutical ingredients (APIs) demand meticulous adherence to compliance and standards. This passage explores the critical role played by compliance frameworks and rigorous standards in ensuring the integrity, safety, and efficacy of active pharma ingredients within the pharmaceutical industry.
The Regulatory Tapestry
Global Harmonization and Regulatory Oversight
The pharmaceutical landscape is governed by a complex tapestry of regulations that vary across countries and regions. Harmonization initiatives, such as the International Council for Harmonisation (ICH), strive to align regulatory requirements globally. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and others worldwide, set stringent standards to govern the development, manufacturing, and quality control of active pharma ingredients. Compliance with these standards is non-negotiable, forming the bedrock of pharmaceutical regulatory frameworks.
Current Good Manufacturing Practice (cGMP)
Central to the manufacturing of active pharma ingredients is the adherence to Current Good Manufacturing Practice (cGMP) regulations. These guidelines, established by regulatory agencies, outline the necessary processes and controls to ensure the consistent quality, safety, and efficacy of pharmaceutical products. Complying with cGMP standards is imperative for API manufacturers, as deviations can result in regulatory actions and compromise patient safety.
Quality Assurance in API Manufacturing
Raw Material Sourcing and Traceability
The journey of maintaining compliance begins with the sourcing of raw materials for active pharma ingredient production. Stringent standards require manufacturers to meticulously select and trace the origin of raw materials, ensuring their quality, purity, and compliance with predefined specifications. The traceability of raw materials is not only a regulatory requirement but a fundamental aspect of maintaining the integrity of the pharmaceutical supply chain.
Process Validation and Control
API manufacturing processes undergo rigorous validation to ensure consistency and reproducibility. Manufacturers must establish and validate critical processes to meet predefined specifications and standards. Continuous process monitoring and control mechanisms are implemented to detect and rectify any deviations promptly. This commitment to process validation contributes to the reliability and quality assurance of active pharma ingredient production.
Analytical Testing and Quality Control
The Crucial Role of Analytical Testing
Analytical testing is a linchpin in maintaining the quality and compliance of active pharma ingredients. Rigorous testing protocols are implemented to assess the identity, purity, potency, and quality attributes of active pharma ingredients at various stages of production. Advanced analytical techniques, including chromatography, spectroscopy, and mass spectrometry, are employed to scrutinize the chemical and physical characteristics of APIs, ensuring they meet the specified standards.
Batch Release and Certificate of Analysis
Each batch of active pharma ingredient undergoes thorough testing before it is released for distribution. A Certificate of Analysis (CoA) accompanies every batch, providing a comprehensive summary of the analytical results and confirming compliance with established standards. This critical documentation serves as a transparent and standardized means of communication between manufacturers, regulatory authorities, and downstream users of APIs.
Compliance Challenges and Continuous Improvement
Navigating Global Challenges
Compliance with global standards poses challenges, especially for multinational pharmaceutical companies. Variations in regulatory requirements and inspection procedures across regions necessitate a strategic and harmonized approach to navigate the global regulatory landscape effectively.
Continuous Improvement Culture
To meet evolving regulatory expectations, the pharmaceutical industry fosters a culture of continuous improvement. This involves proactive engagement with emerging standards, embracing technological advancements, and staying abreast of scientific developments. The commitment to continuous improvement ensures that active pharma ingredient manufacturers remain agile in adapting to changing compliance requirements and industry best practices.
The Future of Compliance in API Development
Emerging Trends and Evolving Standards
As the pharmaceutical industry advances, compliance in API development faces the influence of emerging trends. The integration of quality-by-design principles, advancements in analytical technologies, and the exploration of innovative manufacturing techniques contribute to the evolution of compliance standards. Staying ahead of these trends is crucial for API manufacturers to anticipate and address the challenges of tomorrow.
Sustainable and Ethical Considerations
The future of compliance in active pharma ingredient development extends beyond traditional standards to encompass sustainable and ethical considerations. A growing emphasis on environmentally friendly practices, ethical sourcing of raw materials, and corporate social responsibility is shaping the next frontier of compliance standards in the pharmaceutical industry.
In conclusion, compliance and standards in active pharmaceutical ingredients are paramount to ensuring the safety, efficacy, and integrity of pharmaceutical products. The pharmaceutical industry's commitment to navigating regulatory complexities, upholding quality assurance, and embracing continuous improvement fosters an environment where active pharma ingredients serve as pillars of pharmaceutical excellence. As the landscape evolves, staying at the forefront of compliance trends is not just a regulatory requirement but a commitment to sustaining excellence in API development and, ultimately, advancing global healthcare.